Kidney proximal tubule cell model for safety/efficacy and transporter studies
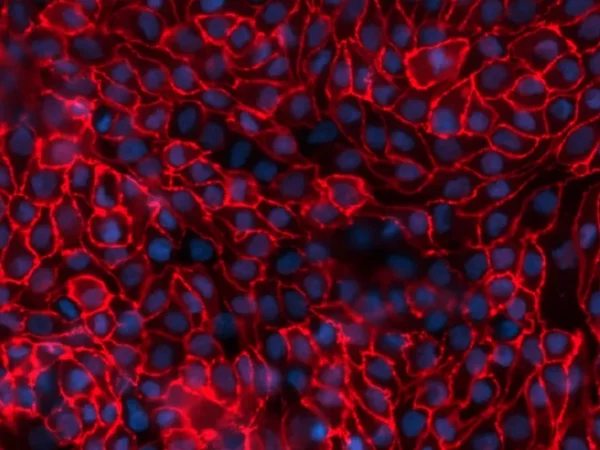
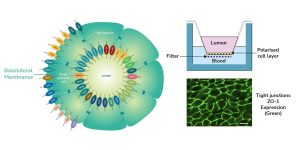
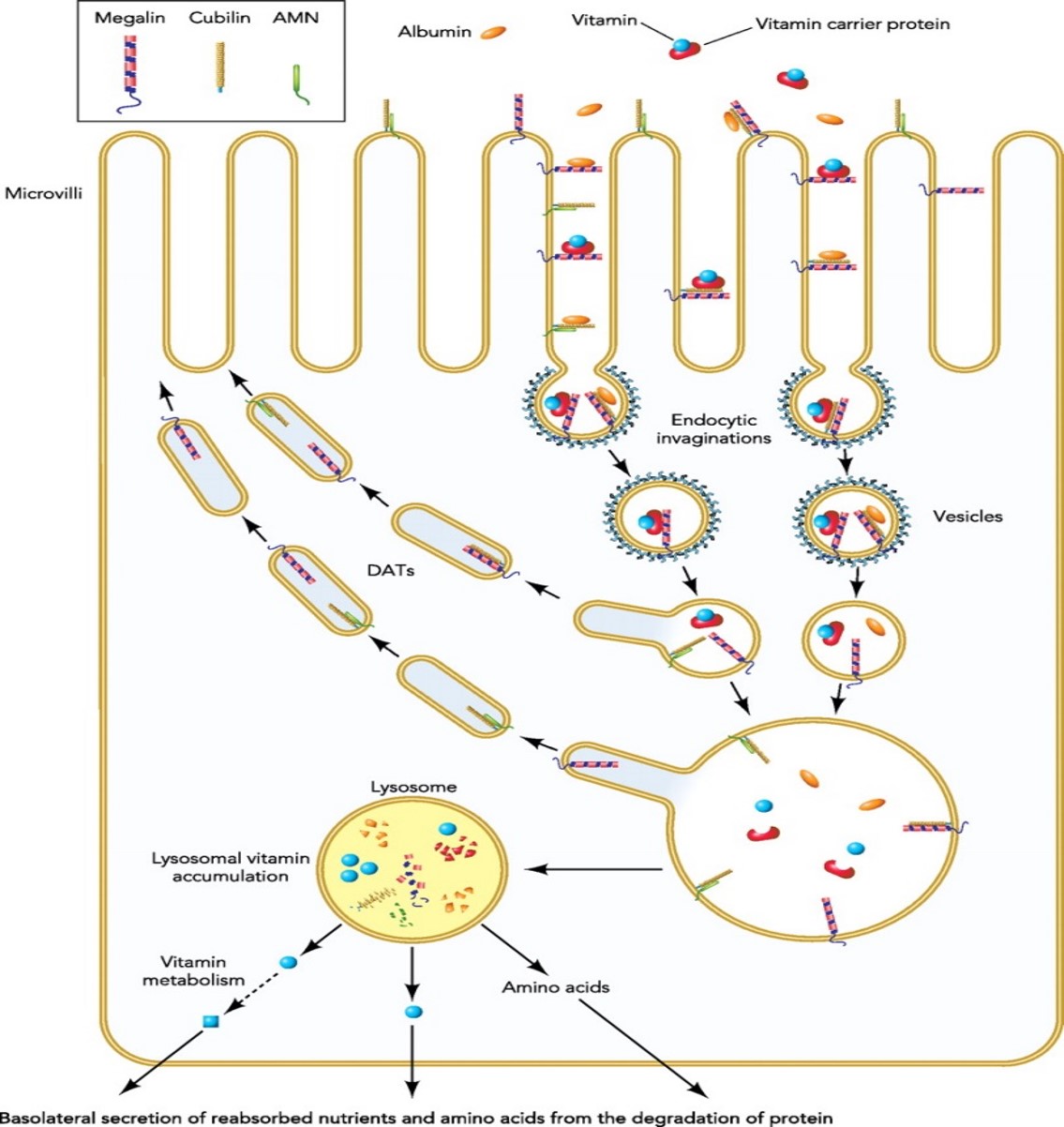
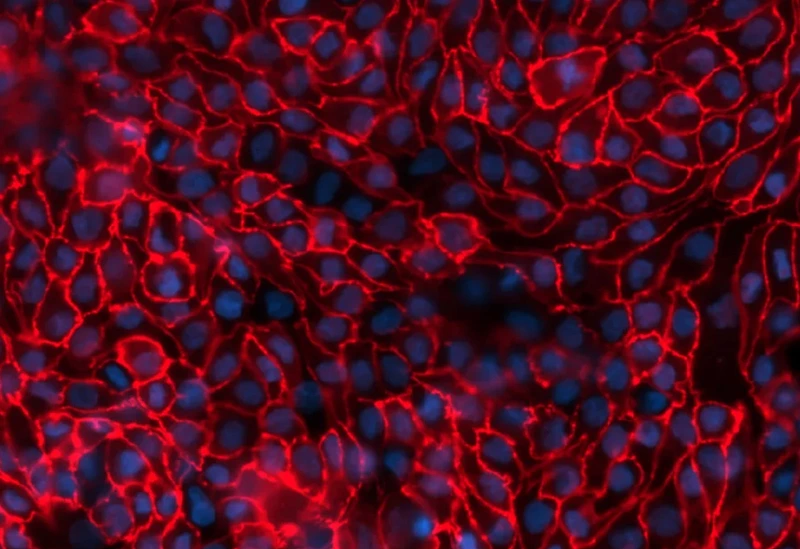
aProximate™ is one of the most advanced, near physiological, in vitro, kidney proximal tubule cell (PTC) models. aProximate™ PTCs are derived from fresh human kidney tissue and grown on Transwells® where they remain as a functional polarised cell layer that forms tight junctions. In contrast to other primary and immortalised kidney proximal tubule cells in culture, aProximate™ PTCs retain high expression of the key transporters involved in drug handling including Megalin and Cubilin, which is ideal for drug transporter studies. Studies with aProximate™ can give you a detailed mechanistic understanding of how new drugs are transported and eliminated through the kidney and how they interact with other drugs prescribed to the target patient population to help mitigate risk of renal toxicity.
Applications
- Drug transporter interactions
- Drug-drug interactions (DDI)
- Species comparison
- Nephrotoxicity assays
- Disease modelling
“The model has been able to closely recapitulate human in vivo proximal tubule functionality and generated valuable insights regarding drug transport by primary human kidney cells. In my opinion, Newcells is the premiere provider of primary human renal cells with physiologically relevant functionality to support toxicity, renal clearance, and renal drug interaction screening.” David Rodrigues, PhD, ADME expert.
Available analytical readouts
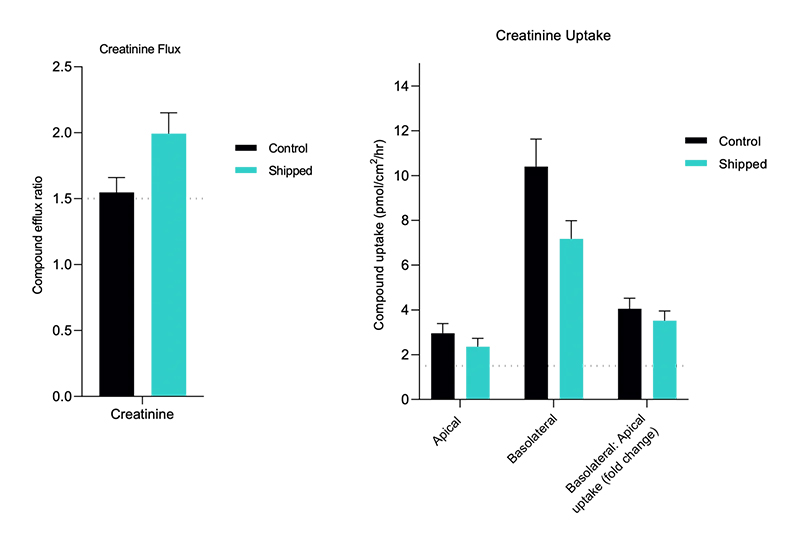
- Flux and net transport measurements
- Measurement of intracellular drug and metabolite concentrations
- Identification of transporter-mediated drug-drug interactions
- Early renal damage biomarkers
- Cell viability