Predictive and investigative kidney toxicity and drug safety evaluation, in vitro
Drug-induced nephrotoxicity is one of the leading causes of drug failure as the prediction of kidney toxicity during new drug development remains inaccurate. Therefore, early detection of is key to reducing both costs and timelines. The FDA recommends in vitro nephrotoxicity assessment of new drugs and biologics prior to in vivo assessments in animal models. Additional regulatory guidance from both the FDA and EMA also recommends the detection of early markers of kidney toxicity such as KIM-1 and clusterin, which can only be quantified in more complex in vitro nephrotoxicity models. Such models are therefore needed for reliably predictive and early evaluation of renal safety profiles. Newcells aProximate™ proximal tubule cell model is the ideal tool for assessing renal toxicity.
Service outputs
-
aProximate™
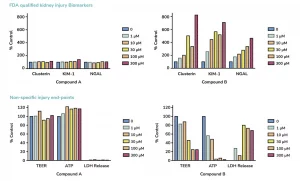
Assessment of FDA-approved biomarkers of kidney toxicity: KIM-1, NGAL and clusterin
Cell viability: ATP, LDH and TEER measurements