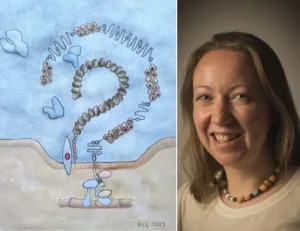Imaging propels our kidney assays to the next level
How state-of-the-art imaging is bringing greater resolution to our aProximateTM large molecule uptake assay
We recently expanded our imaging capability with the purchase of an ImageXpress Pico high content imaging (HCI) system from Molecular Devices. Since then, our technical teams have been busy developing new imaging assays for customers, as well as improving our existing ones. The newest of these is an imaging – based version of our popular assay for monitoring uptake of large molecules via Megalin and Cubulin in proximal tubule cells.
We are seeing a trend towards the development of large molecule therapeutics – oligonucleotides (Including anti-sense oligonucleotides, ASOs), antibody-drug conjugates (ADCs) and the use of albumin as a conjugate to prolong half-life in the body – yet all these and other biologics have the potential to be taken up into proximal tubule cells and cause nephrotoxicity. Testing uptake in a suitable proximal tubule cell model such as the aProximateTM model can be a crucial step in drug development.
The biology of large molecule uptake
Albumin and other large molecules are prevented from entering the kidney tubules by the glomerular filtration barrier. This barrier allows small molecules and ions to pass through due their small size and ensures that other larger molecules are retained in the blood. However, if any large molecules do get through, the kidney has a second line of activity to re-claim and return these back to the blood.
Scavenger receptor Megalin, together with Cubulin, Amnionless and Dab2, form a non-selective protein complex in the apical membrane of proximal tubule cells. This complex binds albumin and other large molecules in the glomerular filtrate and undergoes clatherin-mediated endocytosis to internalise its cargo. From here , the molecules are either transported to the basolateral membrane of the proximal tubule cell to be returned to the blood, or targeted to the lysosomes to be broken down. Large molecule therapeutic compounds including ASOs, ADCs and antibiotics such as aminoglycosides, are also taken up into cells via this route.
The standard Newcells large molecule uptake assay
In our current assay, test compound is incubated with primary proximal tubule cells grown in semi-permeable inserts in multi-well plates. At the end of the incubation period, the cells are lysed, then transferred to a flat-bottomed plate for the measurement of compound uptake through absorbance or fluorescence measurements using a plate reader. Cell lysates can be subjected to mass spectrometry analysis as an alternative. Whole-population measures of uptake give an excellent indication as to whether the test compound is being taken up into proximal tubule cells.
The new, improved imaging assay
By adapting this method to a fluorescence imaging -based one, cells are grown in 96-well plates , and the uptake of compounds observed at the single cell level, improving the resolution of our assay significantly. This assay requires either fluorescent labelling of test compound or the availability of a good antibody to recognise its presence for immunostaining; internalised compound is seen as discrete granules inside the proximal tubule cells. We stain the cell nuclei with Hoechst 33342 and from here, the two-channel image analysis algorithm in the Cell Reporter Xpress (CRX) software identifies and delineates the nuclei, and then detects the granules, assigning each granule to a specific cell depending on proximity to the nucleus. Read-outs are given as number of granules per cell and average (mean) intensity of those granules per cell.
Jonathon Lowe, Associate Scientist in our Kidney team, recently presented a poster on the development of this assay at ISSX in Boston. To read more, (download his poster here).
What’s next?
At Newcells, we have plans to grow our imaging offering further, adding in higher-resolution microscopes and improved image analysis software. Segmenting across multiple fluorescent channels will enable us to not only determine whether test compound is taken up into the proximal tubules, but also where they localise by co-staining with markers for organelles such as lysosomes and endosomes.
Reference :
Molitoris B.A., Sandoval RM., Yadav SPS and Wagner MC (2022). Albumin uptake and processing by the proximal tubule: physiological, pathological, and therapeutic implications. Physiol Rev 102(4):1625-1667.doi:10.1152/physrev.00014.2021. PMID: 35378997.
Share on social media:
Don't miss out on our latest innovations: follow us on Linkedin
Dr Kathryn Garner
12th October, 2023
Kidney