NB win Phase I of CRACK IT Challenge 29: ImmuLiver – securing £99,711 funding
Newcells Biotech are one of three Phase I contract winners of a challenge-led competition that funds collaborations between industry, academics and SMEs to solve business and scientific ‘Challenges’. The NC3Rs challenge (named ImmuLiver), sponsored by Sanofi Pasteur EU, awarded Newcells Biotech Ltd £99,711 to take them through to Phase II of the competition. The aim of the ImmuLiver Challenge is to develop a model of the human liver that is metabolically and immunologically-competent for the routine assessment of Yellow Fever vaccine attenuation.
The UK government are encouraging the development of alternatives to animal testing. The NC3Rs is a UK-based scientific organisation dedicated to replacing, refining and reducing (the 3Rs) the use of animals in research and testing. The development of an in vitro model of the liver sinusoid aims to replace up to 60 cynomolgus macaques that are used annually for the safety assessment of the YF17D viral vaccine. The model could be widely applicable for assessment of vaccine safety and studies of hepatic infection since hepatotoxicity is one of the primary reasons for the failure of new medical entities. Development of a sinusoid model that is a better representation of human liver physiology could reduce animal use considerably, sparing up to 8.7% of the 12 million animals currently used in the European Union for toxicological and other safety evaluations. 1
Currently, existing in vitro liver models lack immune competency.2 A functional liver model that can accurately recreate the complexity of the organ is needed to assess viscerotropism (the tendency to attack or affect the viscera) and replace macaques in the testing of new vaccine products.2
The team at Newcells Biotech Ltd, led by Professor Lyle Armstrong and Dr Colin Brown have proposed an in vitro model of the human liver sinusoid that will respond to yellow fever virus infection in a similar manner to an in vivo liver. The team aims to create a user-friendly system that can be easily introduced into the viral containment systems used during vaccine manufacture and quality control.
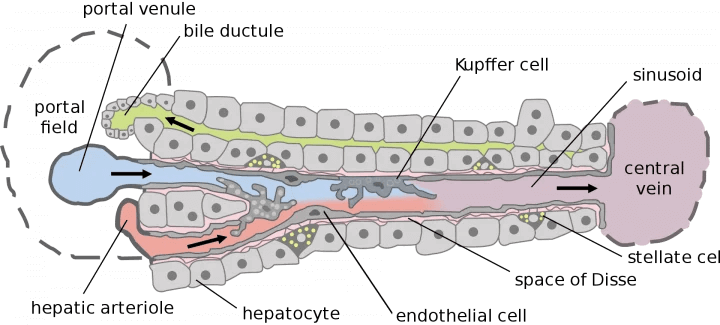
Newcells’ sinusoid model will be constructed entirely from liver cell types generated from human induced pluripotent stem cells (hiPSCs), including hepatocytes and endothelial cells, in addition to those cells of the immune system typically resident or invading the liver during viral infection. The hiPSC-based model will allow the monitoring of the response to yellow fever virus by measurement of the output of cytokines and the synthesis of new viral particles by quantitative RT-PCR, and assess how the infection alters the metabolic performance of the liver sinusoid. The proposed product will be available in 12 or 24 well formats which should permit access into class 3 tissue culture hoods and allow easy sampling of fluid from the apical or basolateral compartments of the transwell culture system.
Yellow fever is a mosquito-borne flavivirus disease affecting human populations in tropical areas of South America and Africa. The WHO estimated that the burden of yellow fever during 2013 was 84 000–170 000 severe cases and around 29 000–60 000 deaths.3 The virus maintains an enzootic cycle in non-human primates (NHPs) and is periodically re-introduced to humans through infected NHPs living nearby. This means the global eradication of Yellow Fever viral infection is not possible, and it remains a significant public health concern.2 However, vaccination can provide those at risk with lifelong protection.3
2https://nc3rs.org.uk/crackit/immuliver
3https://www.who.int/news-room/fact-sheets/detail/yellow-fever
Share on social media:
Don't miss out on our latest innovations: follow us on Linkedin
Newcells Biotech
24th April, 2019
Company News



